ocrevus patient start form
600mg intravenous infusionevery 6. OCREVUS Start Form Ready to get started.
By completing this form you are requesting services on behalf of your patient which may include.

. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. Physicians are encouraged to. 600 mg intravenous infusion every 6 months 23.
OCREVUS Start Form Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions Visit the Site The OCREVUS Co-pay Program Eligible commercially insured patients. Ocrevus ocrelizumab Order Form Please include the following required. 855-838-0623 If Y Medical is the patients choice for Home or On-Site Infusion Services please Call Fax Mail or send an.
Ocrevus ocrelizumab Fax completed form to 8086506487. Date of birth. PATIENT START FORM NEUROLOGY EMAIL.
Ocrevus ocrelizumab Medication Precertification Request. Page 2 of 2 All fields must be completed and return all pages for precertification review Aetna Precertification Notification. Prescription Enrollment Form.
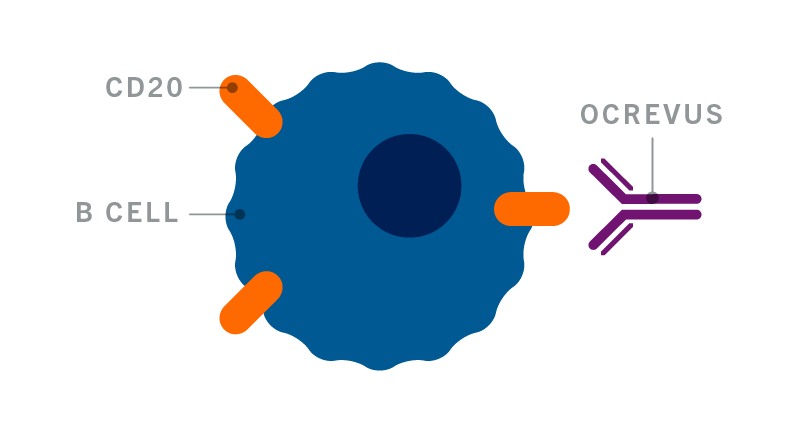
Patient Demographics Insurance Information 2. Prescribers first name. Ocrevus is a prescription medicine used to treat.
OCREVUS is a prescription medicine used to treat. 3 am to pm ET Monday-Friday FAX. 00 College St New Haven CT 00 Please see Indications.
Accredo Additional Medications for Home Infusion Protocol as Per Package Insert. Allergies Weight Patient Phone Diagnosis must include ICD -10 code Multiple Sclerosis _____ Prescription Orders. 300 mg intravenous infusion followed two weeks later by a second 300 mg intravenous infusion 23 o Subsequent doses.
Benefits investigation Benefits reverification approximately 6. Date of Birth. OCREVUS is a prescription medicine used to treat.
ClinicalProgress Notes Labs Tests supporting. OCREVUS START FORM Y Medical Associates Fax Referral To. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary.
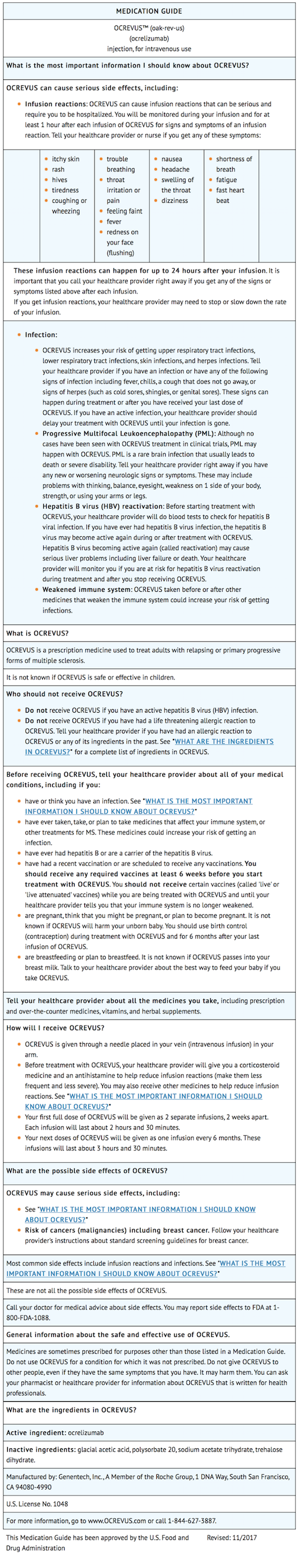
These infusion reactions can happen for up to 24 hours after your infusion. Ocrevus ocrelizumab 02 micron filter must be used during infusion. Prescription Enrollment Form.
O Start dose300 mg intravenous infusion followed two weeks later by a second 300 mg intravenous infusion 23 o Subsequent doses. Patient Street Address. Patients first name.
Swelling of the throat. Start at 30mlhr increasing by 30mlhr every 30 min to a max rate of 180mlhr. There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy.
Duration should be at least 25 hrs. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active.
Ocrevus ocrelizumab Fax completed form to 8883021028. Ocrevus 600mg500ml IV every 6 months 24 weeks. Form are completed Specialty.
It is important that.

Ocrevus Start Form Pdf Fill Online Printable Fillable Blank Pdffiller

Should I Try This Amazing New Drug For Multiple Sclerosis Shots Health News Npr

Accelerated Cure Project Quest Diagnostics And The National Multiple Sclerosis Society Form Research Collaboration To Evaluate Covid 19 Vaccine Efficacy In Patients With Multiple Sclerosis

Pdf A Multiple Treatment Comparison Of Eleven Disease Modifying Drugs Used For Multiple Sclerosis
Ocrevus Access Solutions Patients And Caregivers
A Victory For Multiple Sclerosis Patients Scripps Research Magazine

Attitudes Of Patients With Relapsing Remitting Form Of Multiple Sclerosis Using Disease Modifying Drugs In Montenegro Regarding Covid 19 Pandemic Multiple Sclerosis And Related Disorders

Pdf Health Related Quality Of Life With Diroximel Fumarate In Patients With Relapsing Forms Of Multiple Sclerosis Findings From Qualitative Research Using Patient Interviews

Relapsing Ms And Primary Progressive Ms Treatment Ocrevus Ocrelizumab

Submit Print Or Download Ocrevus Forms Documents Ocrevus Access Solutions
Ocrevus Access Solutions Patients And Caregivers
Ocrevus Access Solutions Patients And Caregivers

Pdf Design Of Trust A Non Interventional Multicenter 3 Year Prospective Study Investigating An Integrated Patient Management Approach In Patients With Relapsing Remitting Multiple Sclerosis Treated With Natalizumab Achim Gass Academia Edu
Ocrevus Side Effects Uses Dosage And More